Levothyrox in France: Bio Equivalency Disproved by Real World Evidence
written by
In 2017 we noticed an increase in the amount of experiences with Levothyrox that were being shared on our
French webpage. This was caused by a change in formulation of this medicine.
In September 2017, we published our findings on this topic in the article Levothyrox: more unsatisfied users since changes in formulation.
Since the publication of that article a lot has happened. The French agency for the safety of health products, ANSM,
published a report stating that the increase in side effects is possibly caused by a thyroid hormone imbalance.
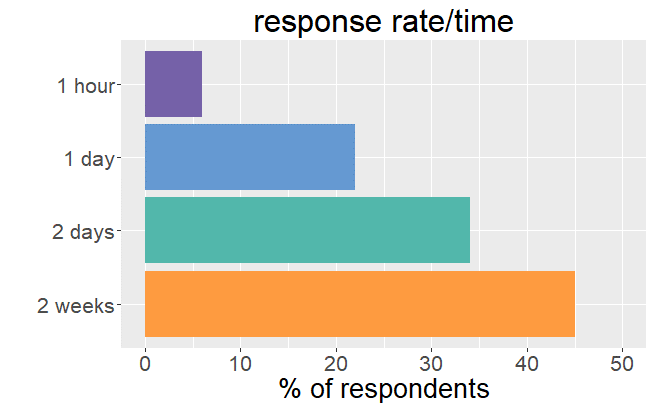
Our survey
 back to the list
back to the list
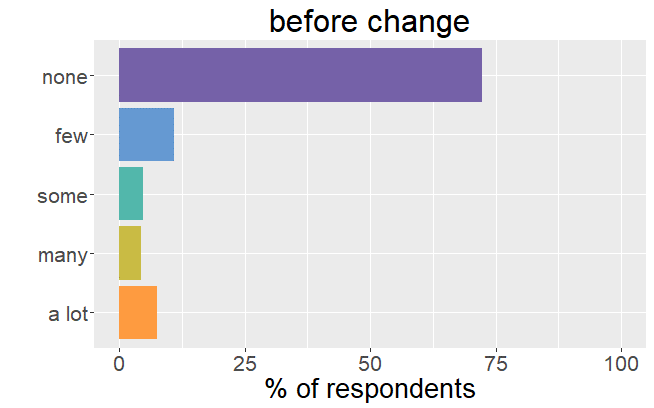
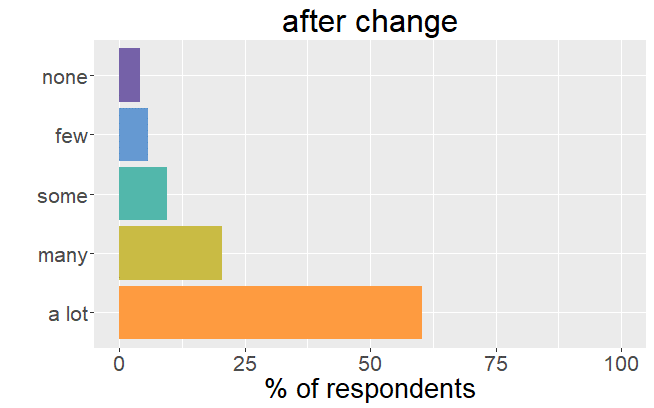
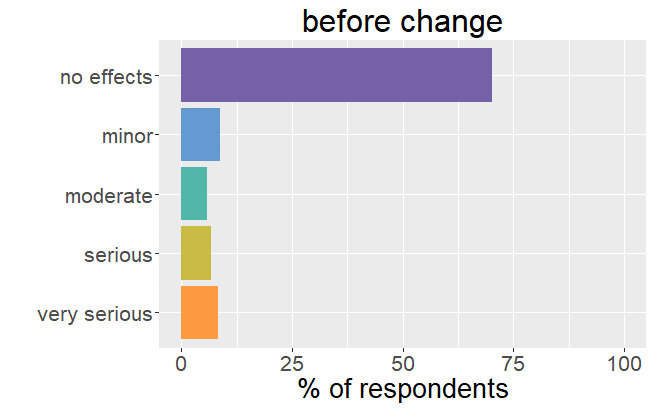
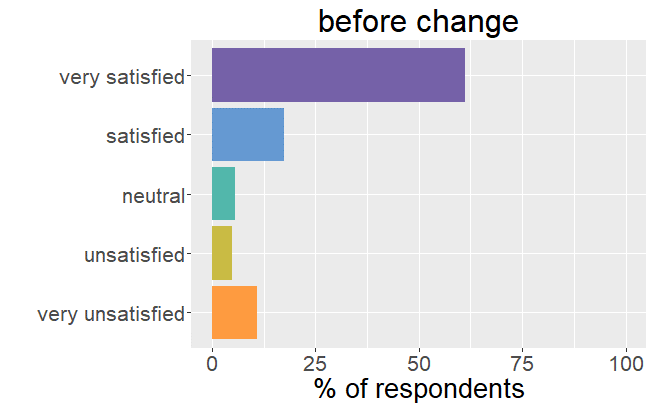
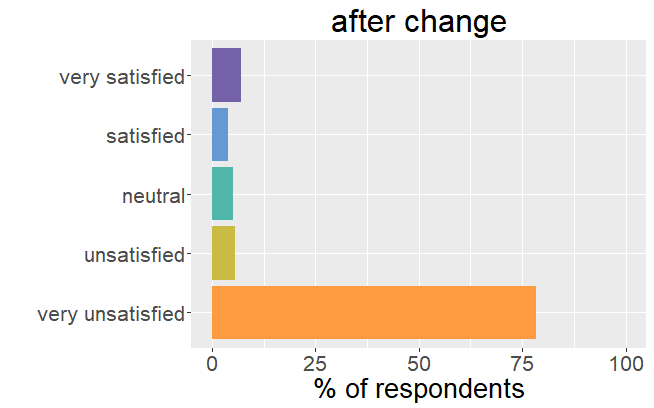
Satisfaction radically changed
Whereas before the change in formulation patients were very satisfied with their thyroid treatment and experienced very few mild
symptoms, it is the complete opposite after the formulation of Levothyrox changed.
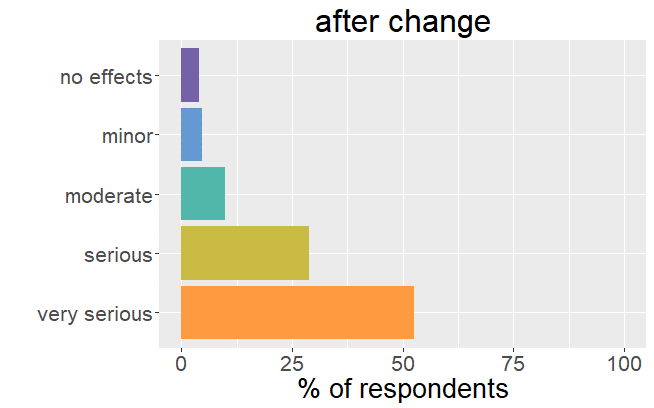
"...fatigue, joint pain..."
"...no side effects..."
"...Loss of balance, tachycardia, fatigue..."
"...very upset, insomnia, depression, big headache, vision disorder, joint problem and great anxiety..."
"Diarrhea, tachycardia, dizziness, foot paresthesia, cramps, depression, life in slow motion, unilateral deafness that worsened..."
It is important to realize that thyroid patients must go through a difficult and lengthy period (till over a year) to find the right adjustments in thyroid hormone.
One can say that patients were well adjusted to their thyroid drug. The change in formulation resulted in an unexpected dysregulation and all the symptoms and side effects that come with it. back to the list
If real world changes are made...
- 44.7% had to adjust their dosage.
- Eventually 79.5% had to change their medication completely, and 8.8% still has the intention to switch.
- The most common were switches to the new options provided by the ministry of health of the country in study; Lthyroxin henning (28.3%), Euthyrox (27%), Lthyroxine serb (4.6%).
But the most telling part is that 32 respondents needed to get medication from other countries to get back to normal.
...the stories tell it all:
"...this treatment deprived me of one year of my life. I'm just starting to get better. I wanted to have a third child, now..."
"I am shocked that we are playing with people's health. I made the connection with my problems via the media in August 2017. Several
doctors treated me like I am crazy and prescribed sedatives (that I have never taken)..."
"Dizziness exhaustion hair loss nausea disappeared in 8 weeks but abdominal pain in the pancreas and loss of appetite and a remainder
of chronic fatigue persisted until mid-February. I found my "normal" condition at the end of 5 1/2 months..."
If we only knew...
Patients are very frustrated by the lack of communication. Three out of four patients were not informed of the change of formulation.
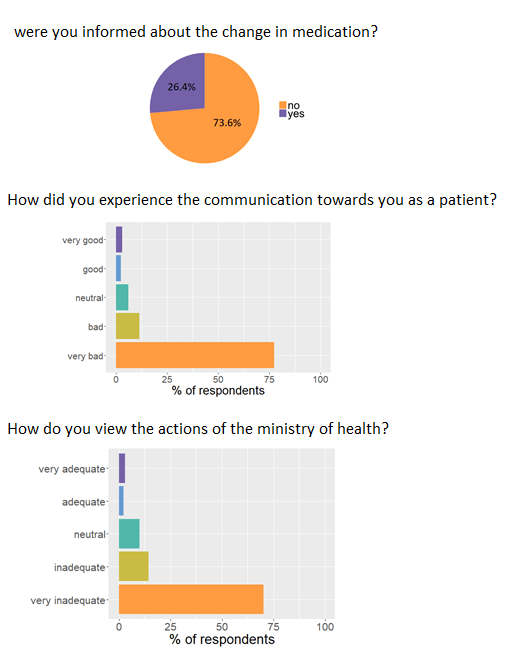
"...very angry by the denial of doctors and medical health authorities, [they have] more confidence in the labs. Unknowingly we were
used as guinea pigs and we were made to be depressed. I add that my blood tests remained normal, except my transaminases which had
doubled..."
"The general reactions of the medical profession (with some exceptions, fortunately) have been and still are inappropriate and
contemptuous of the pain endured and the uncertainty for the near future. Nothing is solved."
Respondents also remarked that they did not know or weren’t provided the option to switch to new medication when this option was made
available by the ministry of health. Because of these issues the patients feel like communication is lacking and the response of the
ministry of health is very inadequate.
"I would like to go back to the old formulation but my doctor does not want this, I have the feeling of not being heard..."
"My life was stolen because of this change of Levothyrox I am 60 years old and too much physical and moral suffering my body is
suffering I became a bedridden"
"...the reaction of the Ministry of Health was catastrophic. But what is most shocking is the reaction of doctors (informed) and
specialists who doubt our words and our difficulties! I even heard from an endocrinologist friend that this was a collective psychosis !!"
Patients are angry about this lack of information and feel like they are not taken seriously. Out of these patients 91 respondents
have now started a (collective) judicial process.
Conclusion
The switch in formulation of Levothyrox has had great impact on a lot of people’s lives. Severe side effects manifest themselves,
leaving the patient to search for a solution. Some are helped by adjusting their dosage but others need to resort to new medications,
sometimes even imported from other countries. What might be even the biggest complaint is the lack of communication and understanding.
This situation shows that even though minor changes, thought to be of no consequence, can actually cause major changes in patients’
health. Obviously, bio equivalence studies do not predict the outcome in patients and should be regarded insufficient in determining
real life responses.